How YCANTH Works
** Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14(10):42-47.
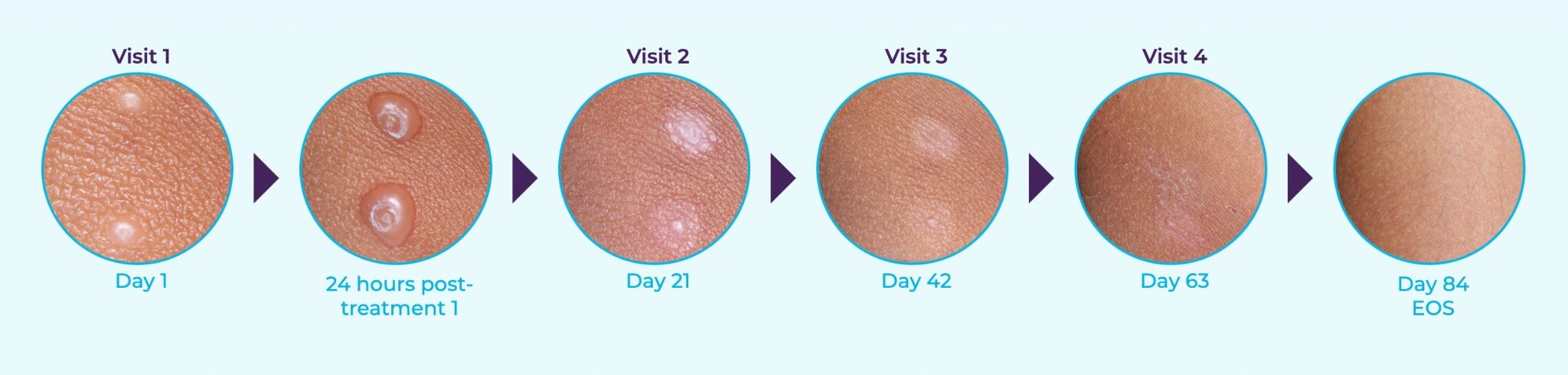
YCANTH Phase 3 Clinical Trial Results*
The road to prescribing is simple.
Complete The Survey
Answer a few easy questions and we’ll let you know whether you may be eligible to provide YCANTH treatments to your patients.
Schedule A Chat
Qualifying physicians can speak to a team member, who will set you up with a brief session on how to administer the treatment in-office. Verrica is committed to providing support to ensure prescribers receive an optimal training experience.
Start Prescribing
Once you’ve completed YCANTH’s safety training, it will be shipped to your practice to begin dispensing to patients.
Our dedicated team is available to answer any questions. We’re here to help.
Who Should Be Treated with YCANTH?
Pediatric patients two years of age and older, as well as adults who have a confirmed case of molluscum contagiosum are eligible to receive treatment.
Why Treat Now?
Molluscum is not only highly contagious, but can also cause discomfort. It affects approximately 6 million people in the United States,1,2 and its prevalence appears to be increasing across all age groups worldwide.3 Outbreaks may take several months up to years for spontaneous recovery, during which time patients’ lives may be disrupted.
2: IQVIA Market Sizing Study. July 2018.
3: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10453394/#B5
Number of people affected by molluscum contagiosum in the United States.
1. Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130-136. doi:10.1093/fampra/cmt075
2. US Census Bureau. 2021 American community survey 1-year estimates. Accessed May 24, 2023. https://data.census.gov/table?q=children&tid=ACSST1Y2021.S0901
Reasons to Treat4,5

Cannot attend school
Absent from daycare
Unable to play with other children
Disrupted schedule for caregivers
4: Silverberg NB. Pediatric molluscum: an update. Cutis. 2019 Nov;104(5):301-305;E1;E2. PMID: 31886783.
5: Kwong, P., Hebert, A., Utley, C., & Olivadoti, M. (2021). The Hidden Impact of Molluscum Contagiosum: A Survey of Caregivers’ Experiences with Diagnosis, Treatment, and Impact on Quality of Life. SKIN The Journal of Cutaneous Medicine, 5(4), 363–371. https://doi.org/10.25251/skin.5.4.5
Now that there is an effective, FDA-approved treatment that can easily be given in-office, prescribers can offer patients a better path forward.
Understanding Molluscum Contagiosum
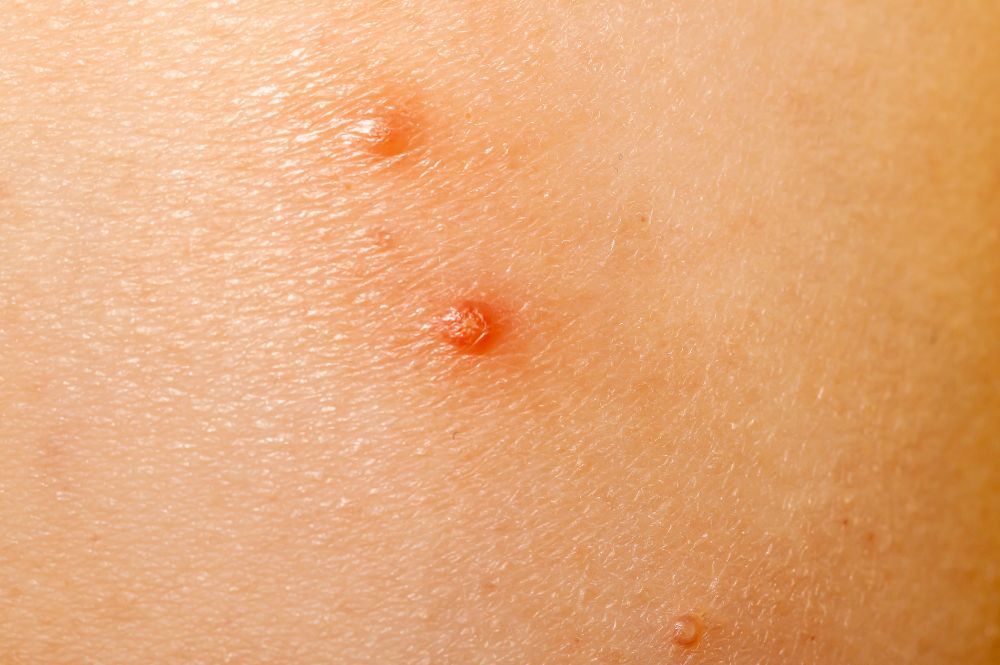
What It Is
Molluscum is a viral skin infection that causes the appearance of white, pink, or flesh-colored firm round bumps. The bumps, which may itch or become irritated, may present as a single lesion or in clusters. Lesions can appear anywhere on the skin, including the face, neck, stomach, arms, legs and genital area. It is transmitted via skin-to-skin contact, and is most common among children under 10 years old. It affects millions of children each year, with the highest prevalence occuring in preschool children (aged 1-4).6
Current Standard of Care
Prior to now, one therapeutic approach was often to just “wait and see”, with physicians simply monitoring the appearance of bumps until spontaneous resolution occurred — a process that could take an average of 13 months, up to 5 years. Molluscum can spread by skin-to-skin contact, through fomites and by autoinoculation. It can spread to siblings through households and in classrooms. It can be highly symptomatic and cause itching, pain and redness.
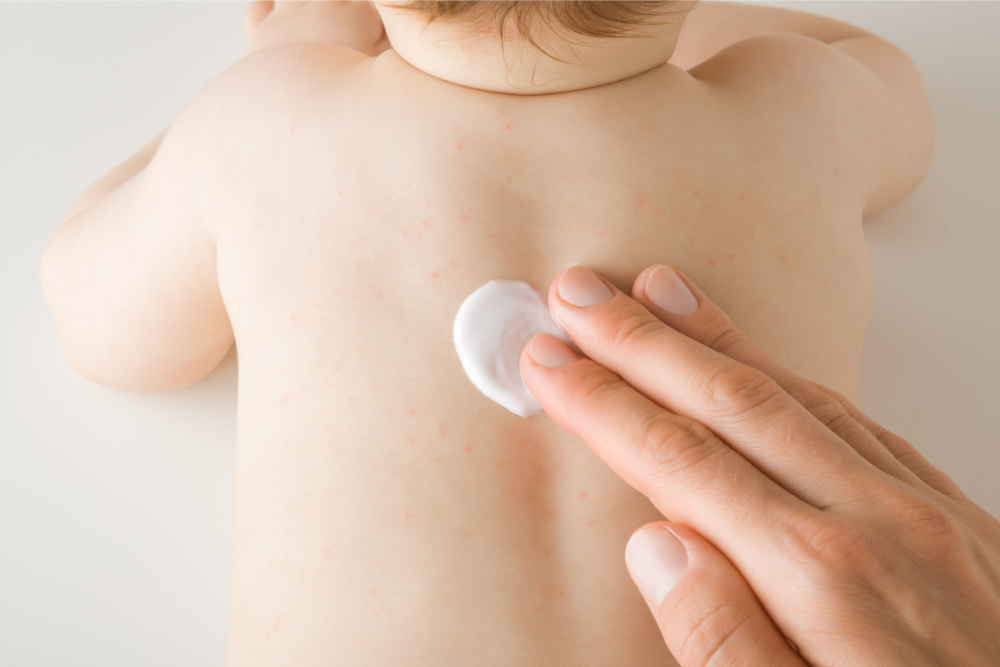

Visible Results
The active ingredient in YCANTH, cantharidin (0.7%), targets each lesion and acts as a vesicant, causing a blister to form on the bumps. Applied every three weeks as needed, patients receive the topical application in-office, then remove it after 24 hours with soap and water. In clinical trials, YCANTH provided clearance for many patients within 12 weeks7. Finally, it’s time to give patients the solution they deserve.
7: CAMP-1 46% vs 18% (P<0.0001); CAMP-2 54% vs 13% (P<0.0001)
Frequently Asked Questions
Who should become a prescriber?
You should answer a few easy questions to become a certified prescriber if:
- You are a licensed pediatrician who sees molluscum patients whether routinely or sporadically
- You are a healthcare professional who treats skin disorders like molluscum contagiosum
- You are interested in learning more about an in-office therapy
- You are willing to participate in a brief training program
How do I get YCANTH?
Are there any costs to become a prescriber?
No, qualified prescribers do not pay for the training program.


