About The Drug
YCANTH is an FDA-approved topical solution (cantharidin) 0.7% for the treatment of molluscum contagiosum. It is approved for use on adults, and pediatric patients two years of age and older.1
What Are The Benefits?
- Reduces disease progression and transmission, including autoinoculation
- Reduces discomfort and itching
- May help alleviate anxiety in caregivers
- Prevents exacerbations of comorbidities, such as atopic dermatitis, and may help prevent bacterial infections
- May reduce the negative impacts on quality of life due to scarring, bullying, and exclusion from activities
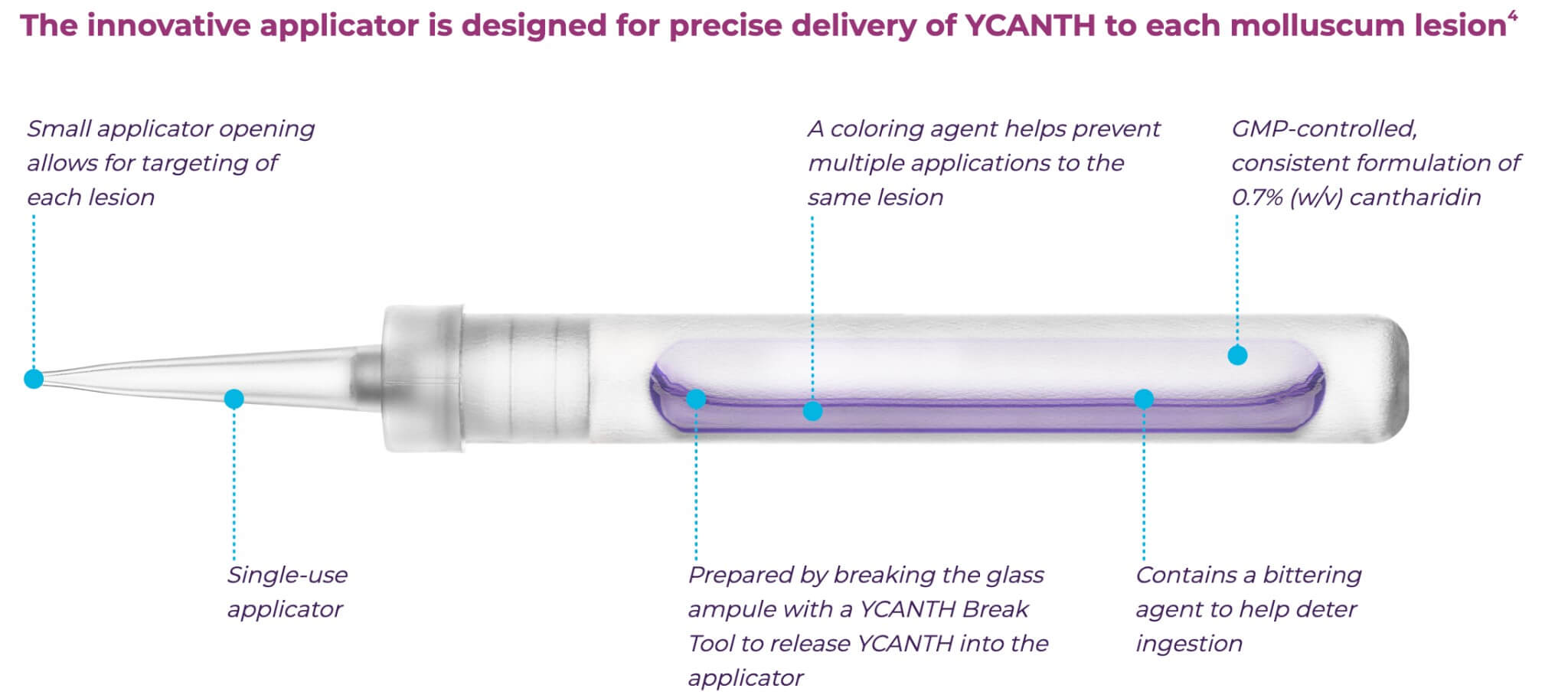
YCANTH Defines The Treatment Experience
It may be administered every 3 weeks as needed, and applied only to lesions that are practically and medically safe to treat. YCANTH should be removed with soap and water 24 hours after treatment. If severe blistering, severe pain, or other adverse reactions occur, remove YCANTH prior to the recommended 24 hours after administration. Do not cover treated lesions with bandages.1
The solution is clear — and so is patients’ skin. Take our brief survey to see if you are a good fit to join other prescribers already helping to tackle molluscum.
1: https://verrica.com/pi/ycanth
2: Silverberg NB. Cutis. 2019;104(5):301-305.
3: American Academy of Pediatrics. Red Book. 2021:535-537
4: van der Wouden JC, et al. Cochrane Database Syst Rev. 2017;5:CD004767