Safety and Efficacy
YCANTH provided complete clearance of baseline and new lesions within 12 weeks
Complete clearance: pooled CAMP-1 and CAMP-2 data (ITT population)*
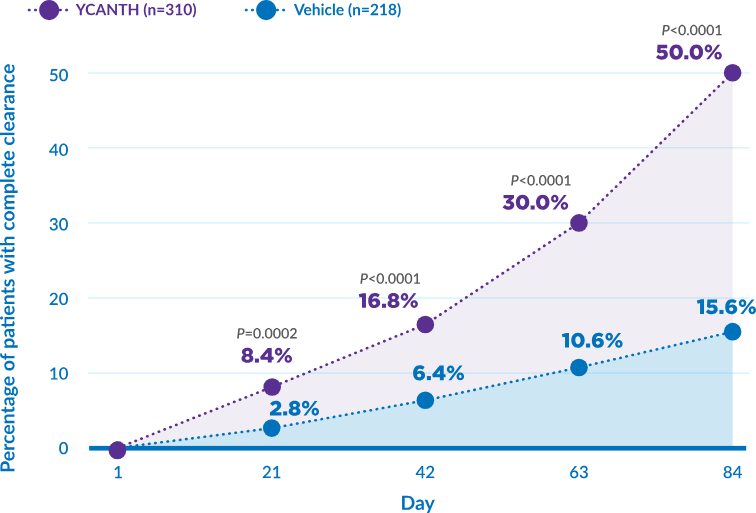
- CAMP-1 statistical significance at days 21, 42, 63, and 84. CAMP-2 significance at days 42, 63, and 84. Read Study.
- *The intent to treat (ITT) population included patients randomized to receive either YCANTH or vehicle. Read Study.
- CAMP-1/CAMP-2=Cantharidin Application in Molluscum Patients-1 and -2; ITT=intent to treat.
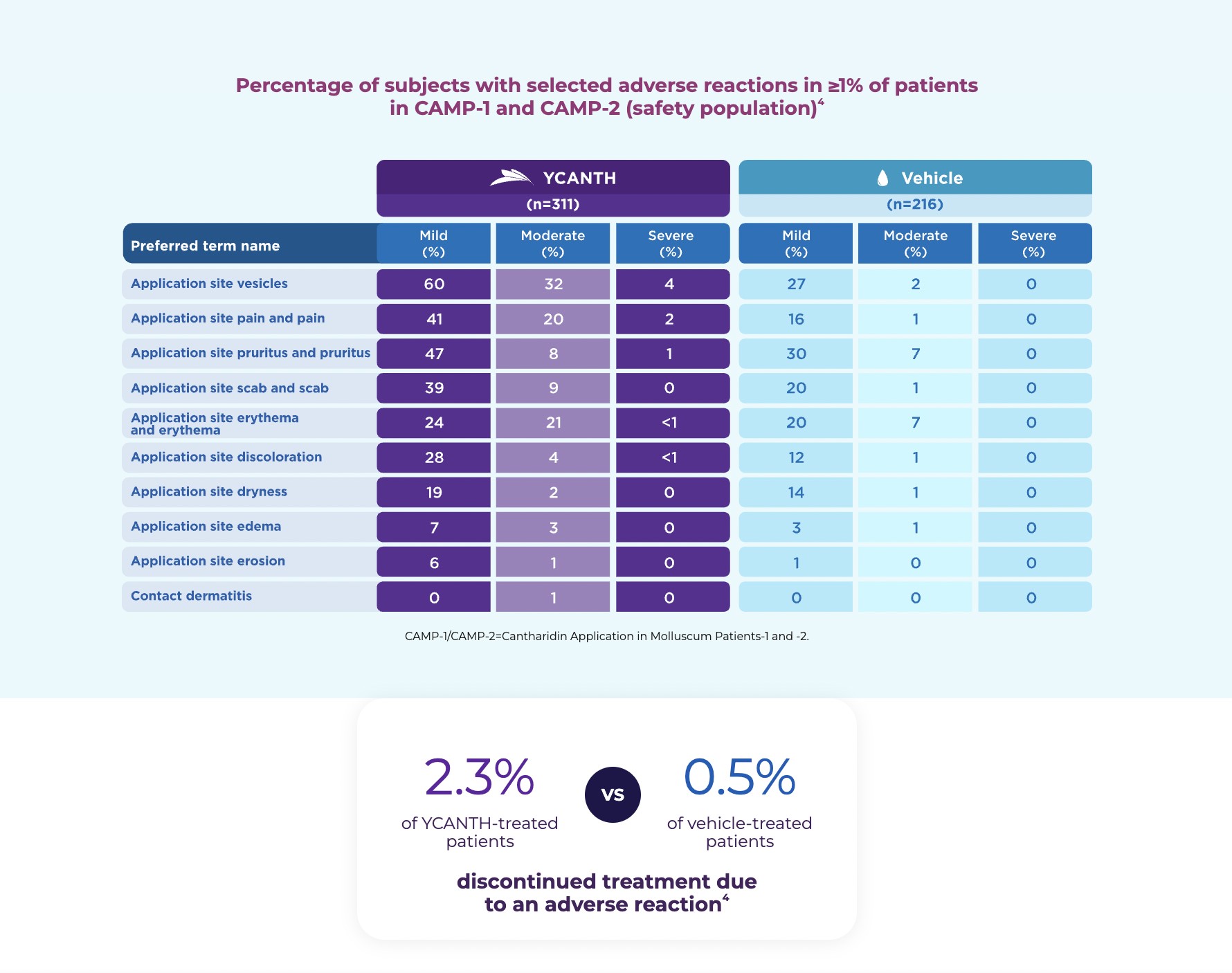
Adverse reactions were primarily mild to moderate local skin reactions at the application site and included vesiculation, pain, pruritus, discoloration, and erythema. See additional Important Safety Information throughout.
The primary efficacy endpoint was the percentage of patients with complete clearance of baseline and new lesions by day 84 with YCANTH vs vehicle:
| CAMP -1 (46% VS 18%) (P<0.0001) |
CAMP -2 (54% VS 13%) (P<0.0001) |
You may find full prescribing information here.
What you should know before administering YCANTH1
- Verrica is committed to providing a training program to ensure an optimal experience.
- Counsel your patients to avoid contact with the treatment areas, including oral contact.
- YCANTH is flammable; avoid fire, flame, or smoking near lesion(s) during treatment.
- YCANTH is not for oral, mucosal, or ophthalmic use.
YCANTH phase 3 clinical trial results
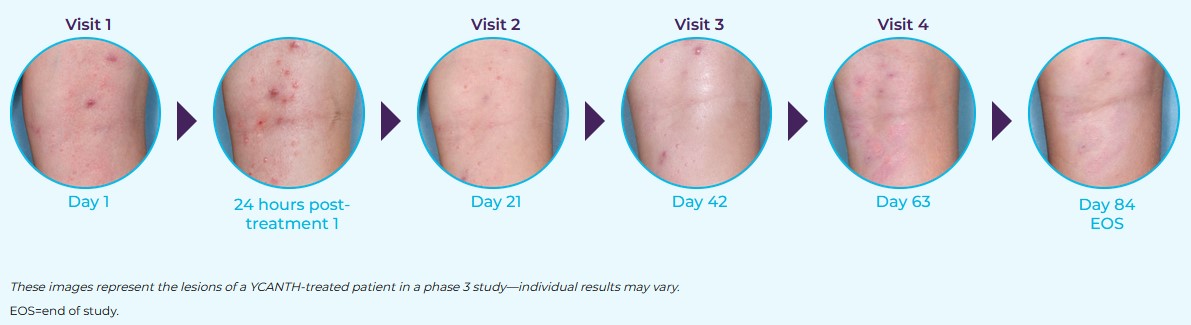
The CAMP-1 and CAMP-2 phase 3 clinical trials enrolled 528 patients (CAMP-1: N=266; CAMP-2: N=262) with molluscum aged 2 years and older. Patients with active or a history of atopic dermatitis were included in the studies. Study drug (YCANTH or vehicle) was administered topically to all baseline and new lesions approximately every 21 days until complete lesion clearance or up to 4 applications.2,4 Assessments of expected application-site reactions, including incidence/size of blisters, erythema, pain, pruritus, and edema, were conducted at each visit through patient/caregiver report, as well as at 24 hours, 7 days, and 14 days after each treatment.2